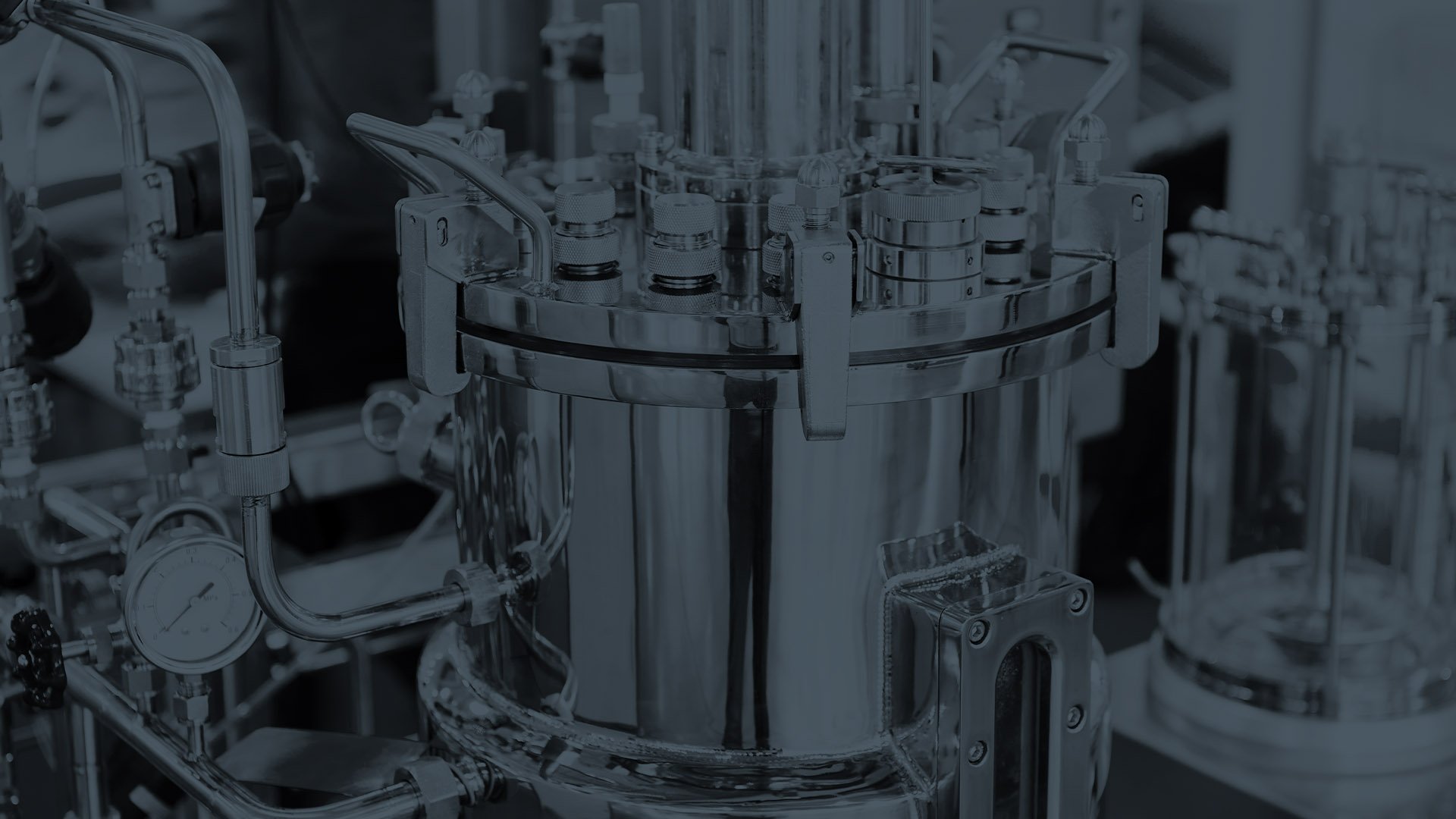
Customised viral vector manufacturing for cell and gene therapy
Who we are
We are a New Zealand-based lentivirus manufacturer offering affordable, customised viral vectors for cell and gene therapy with world class quality.
Cost effective
New Zealand based
World class quality
Why?
We want to provide patients worldwide with easy access to affordable treatments for cancers and genetic disorders.
What?
We specialise in customized lentiviral vectors production and purification for cell and gene therapy.
How?
BioViros team counts years of experience in cell biology, CAR-T therapy, virus production, biologics purification and science commercialisation. Supported by Bridgewest Ventures and Callaghan Innovation, we strive to innovate and improve lentivirus vector manufacturing, and make the bench to bed journey faster and cheaper for people that need life-saving treatments, while keeping the high-quality standards.
Our Partnerships
In Partnership
BioViros is proudly supported by Bridgewest Ventures NZ LP. Bridgewest Ventures is part of the Bridgewest Group and has a long track record of successfully incubating and launching major global innovation-led companies.
Bridgewest’s deep technology experience is wide-ranging and includes drug discovery and deep research into health technology, with expertise in novel CAR T-cell platforms.
Bridgewest Ventures leverage their extensive connections, international reach, and commercialisation experience to transform innovative opportunities into world-class companies.
Fostering innovation in NZ
We are delighted to be part of Callaghan Innovation’s Technology Incubator Programme.
Callaghan Innovation is New Zealand’s innovation agency. It activates innovation and helps businesses grow faster for a better New Zealand. The government agency delivers a range of innovation and R&D services to suit each stage of business growth.
Callaghan Innovation staff, including more than 200 of New Zealand’s leading scientists and engineers, empower innovators by connecting people, opportunities, and networks and providing tailored technical solutions, skills and capability development programmes, and grants co-funding.
Advancing Healthcare
Our close partnership with BioOra is a cornerstone of our commitment to advancing healthcare.
BioOra specializes in automating the manufacture of CAR T cells for personalized cancer therapeutics, offering an affordable, high-throughput pathway to market. As their trusted supplier of custom viral vectors for cell and gene therapy, BioViros ensures world-class quality in this essential component.
Together, we are driving innovation and making a significant impact on patients' lives through cost-effective, cutting-edge treatments.
Get in touch
Get in touch for a FREE assessment of your research and clinical needs.






